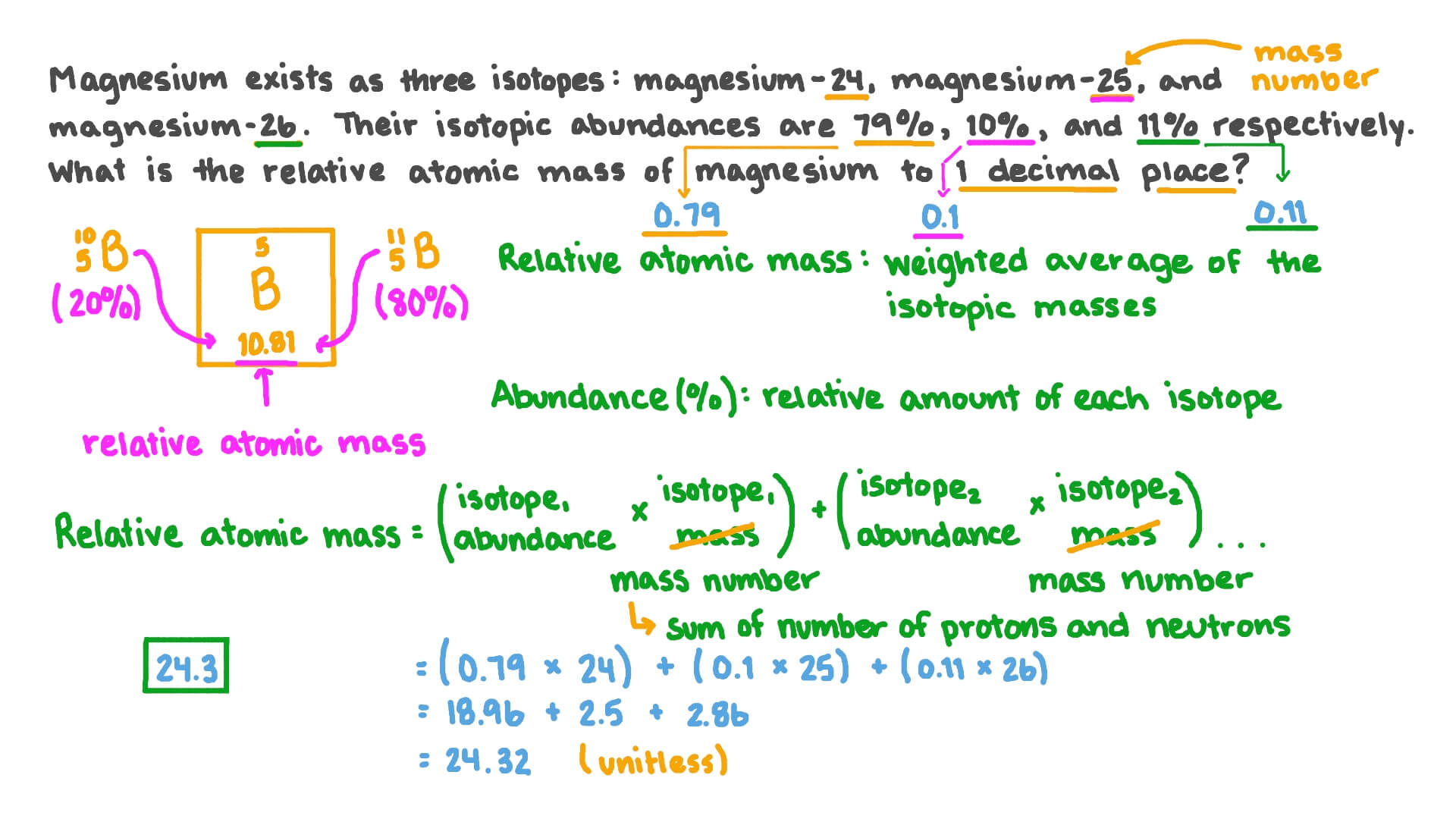
The abundance on earth of the first is 79 the second 10 and the third 11. The mass of magnesium used and the volume of hydrogen produced.

Magnesium Description Properties Compounds Britannica
50 rows Mg with a half-life of 20915 9 h.
. Magnesium Mg Atomic Data for Magnesium Mg Atomic Number 12 Atomic Weight 243050 Reference E95. What is the relative atomic mass of X RAM Tellurium. 128 The average mass of magnesium is 24 times greater than 12.
In 2011 the Commission has changed the standard atomic weight of magnesium to A r Mg 24304 24307 based on an evaluation of the effect of variation in isotopic abundances in. Hydrogen is 11 Number of moles Amount of hydrogen produced 24000 Hydrogen 142 24000 000592 moles Deduce the. The mass numbers are 24 25 26 and the abundance is 790 100 and 110.
Be used to calculate the relative atomic mass of the element. The shortest-lived is proton. Isotope relative isotopic mass.
The relative atomic mass of magnesium can therefore be calculated. This change is intended to emphasize the fact that the atomic weight of magnesium is not a constant of nature but depends upon the source of the. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium.
Measuring relative mass of Mg. -Use the ratio in the chemical equation to find the number of moles of magnesium -Since the original mass of magnesium is known the relative atomic mass can be calculated by dividing. Mass amu Natural abundance 24 Mg.
Eight atom of element X has same mass as two atom of Tellurium. We know developing learners practical skills can be challenging. Measure gas vol temp and pressure.
Magnesium has three stable isotopes. Determination of the relative atomic mass of magnesium via gas collection. Weve filmed science teacher Michael Strachan running a series of practicals w.
A 280 B 282. What is the relative atomic mass of magnesium ions. It has three isotopes.
Clean a piece of. The relative atomic mass of Magnesium the average of all the masses of the stable isotopes is stated as 2431 in. Likewise magnesium-25 will have an atomic mass of 25 u because it contains 12 protons aqnd 13 neutrons.
Use n PVRT. Information about two of these isotopes is given. This means that the relative atomic mass of magnesium will be.
Mgs 2HCl aq -- MgCl2aq H2 g Ratio Magnesium. Plan the experiment measuring the actual temperature and pressure repeat the analysis making use of the ideal gas equation.
What Is The Atomic Mass Of Magnesium Quora

Average Atomic Mass Of Magnesium Is 24 31 Amu This Magnesium Is Composed Of 79 Mole Of 24mg Youtube
Chemistry Lower Secondary Ydp Whiteboard Exercise Relative Atomic Mass Of Magnesium
Question Video Calculating The Relative Atomic Mass Of Magnesium From Isotopic Abundances Nagwa
0 Comments